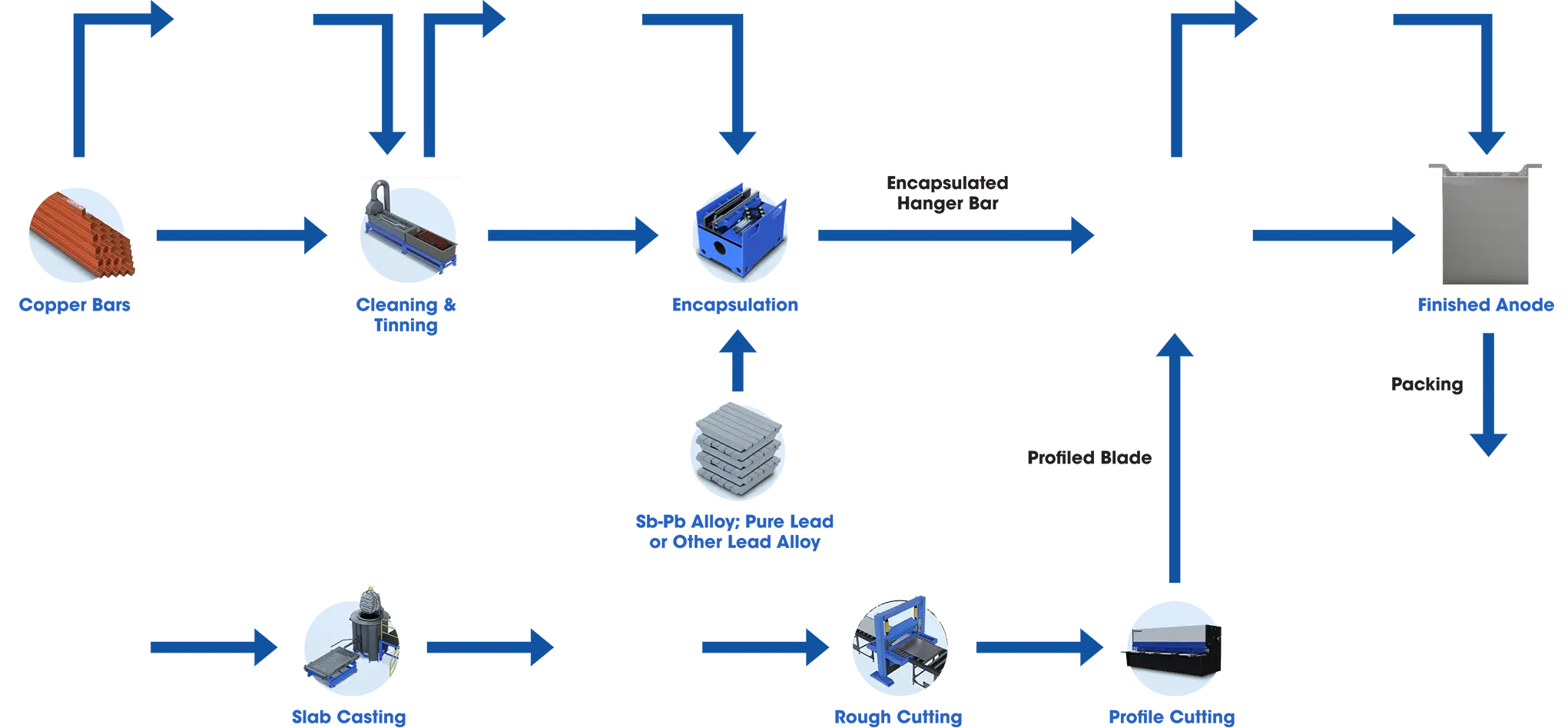
When in use, pure lead cannot support its own weight and will creep. This creep will break the lead oxide layer on the anode surface, causing exposure of fresh lead to oxidisation and corrosion. Alloying of the lead increases the mechanical strength during use, preventing creep and thus the cracking of the oxidised surface layer and maintaining the anode shape. Lead antimony, lead tin antimony and lead silver were used for many years in copper and zinc electrowinning as alloys to provide the desired mechanical properties. With the advent of solvent extraction and electrowinning systems to produce high-purity cathode deposits, the need arose for much lower lead levels in the cathode deposit. As such, alloys with calcium were developed to reduce the level of lead deposit. As calcium lead alloys are relatively weak, tin is added to increase mechanical strength and decrease creep. Tin addition also reduces passivation when the power is interrupted.
Silver Lead Anodes are used in Zinc Electrowinning. Silver reduces the rate of corrosion over the service life. Typically, the silver content is between 0.4% and 1%. To improve the mechanical properties of the Ag-Pb anodes, allowing elements such as strontium, barium and calcium can be added.